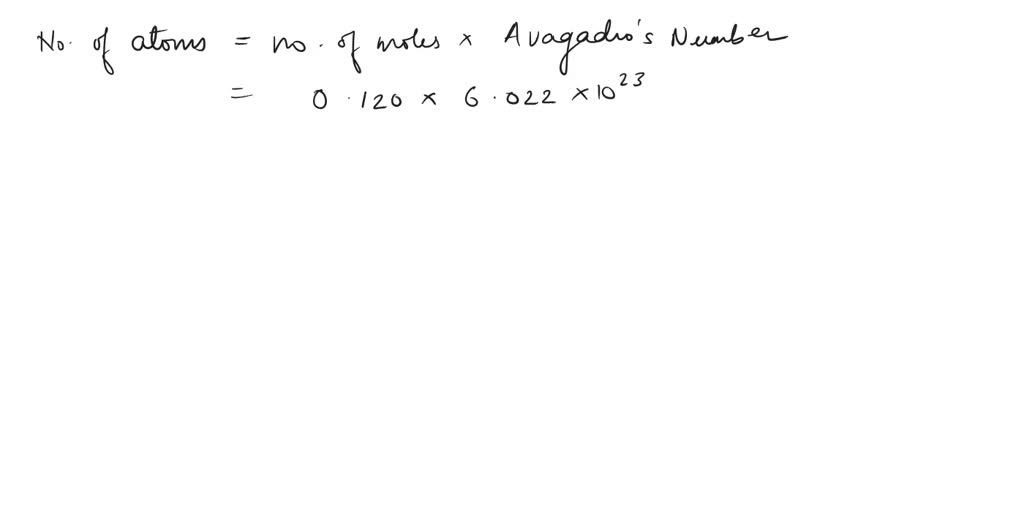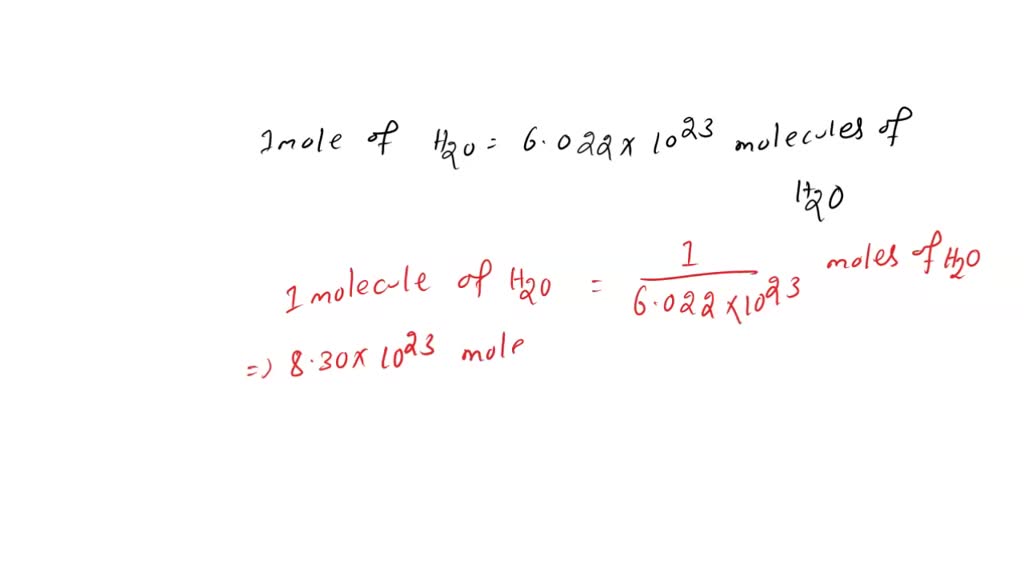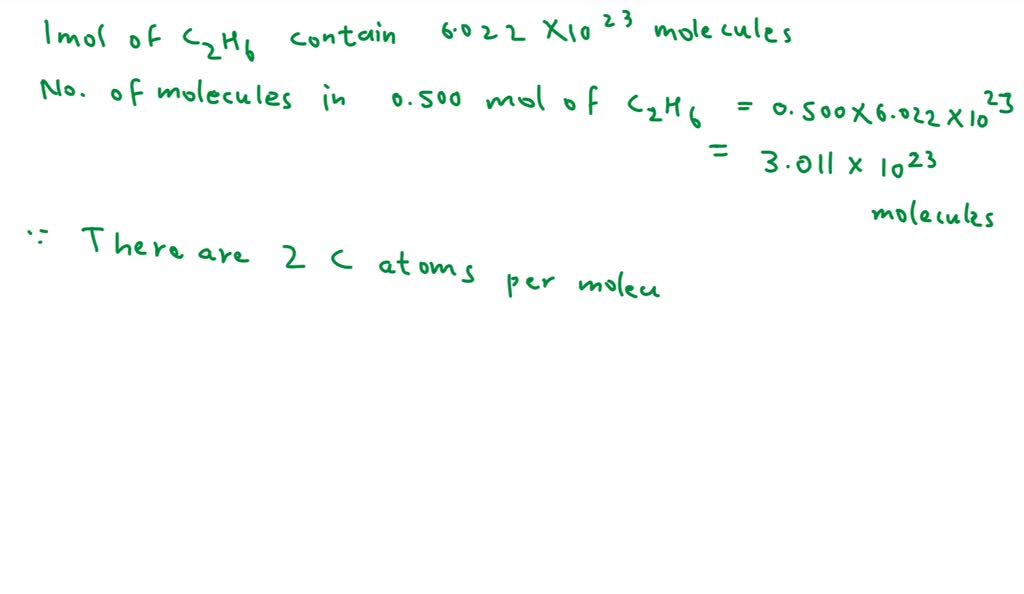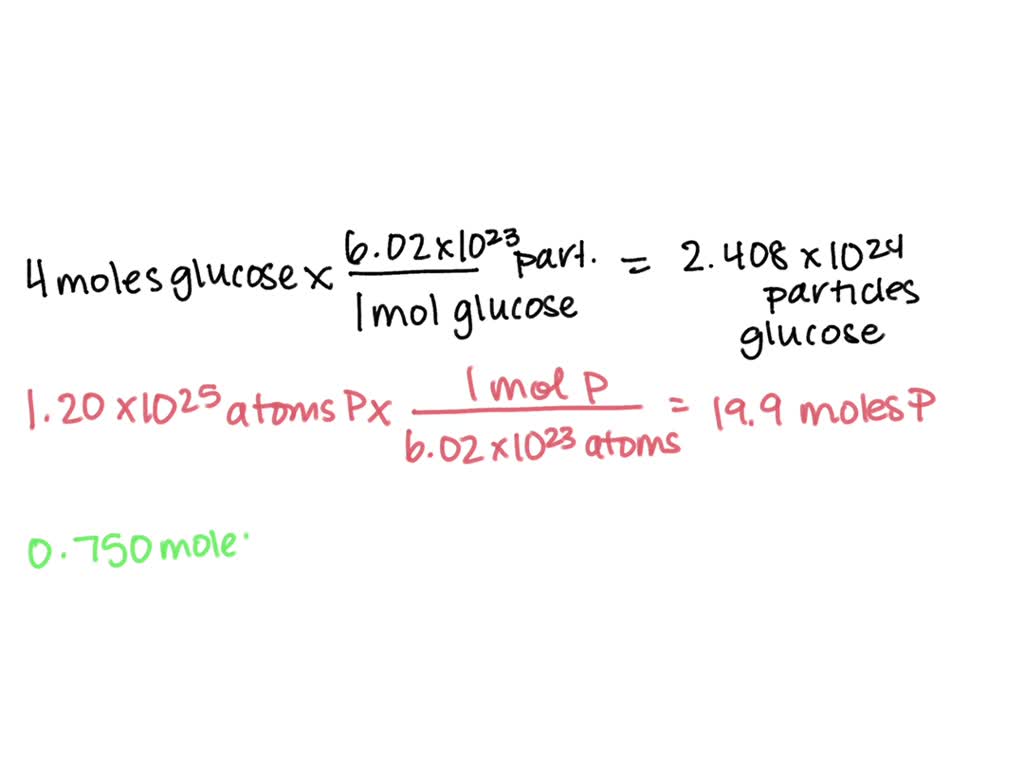Convert 3 01 X 1023 Molecules Of C2H6 To Moles - Online molecules to moles calculation. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. So, there are approximately 0.50 moles of. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. Use this simple science molecules to moles calculator to calculate mole. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Convert 3.01×10 23 molecules of c 2 h 6 to moles. One mole of any substance contains 6.02×10 23 atoms/molecules. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. 1 mole contains 6.022× 1023 number of molecules.
To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. One mole of any substance contains 6.02×10 23 atoms/molecules. Convert 3.01×10 23 molecules of c 2 h 6 to moles. Online molecules to moles calculation. Use this simple science molecules to moles calculator to calculate mole. 1 mole contains 6.022× 1023 number of molecules. So, there are approximately 0.50 moles of.
So, there are approximately 0.50 moles of. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Convert 3.01×10 23 molecules of c 2 h 6 to moles. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. Use this simple science molecules to moles calculator to calculate mole. One mole of any substance contains 6.02×10 23 atoms/molecules. 1 mole contains 6.022× 1023 number of molecules. Online molecules to moles calculation.
SOLVED Calculate the number of atoms in 0.120 moles of Na. Calculate
Use this simple science molecules to moles calculator to calculate mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50.
Solved How many moles of CO2 are produced when 3.01 x 1023
So, there are approximately 0.50 moles of. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. Use this simple science molecules to moles calculator to calculate mole. 1 mole contains 6.022× 1023 number of molecules. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x.
SOLVED B How nHY 1 '24 1 moles 8 m 2 molecules water molecules 3 are
One mole of any substance contains 6.02×10 23 atoms/molecules. Online molecules to moles calculation. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Convert 3.01×10 23 molecules of c 2 h 6 to moles. So, there are approximately 0.50 moles of.
How many moles of carbon atoms are there in 0.500 mole of C2H6? 4.00
1 mole contains 6.022× 1023 number of molecules. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Use this simple science molecules to moles calculator to calculate mole.
Solved How many moles of CO2 are produced when 3.01 x 1023
To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. One mole of any substance contains 6.02×10 23 atoms/molecules. So, there are approximately 0.50 moles of. Online molecules to moles calculation.
SOLVED 1 mol = 6.02 x 1023 particles (atoms or ions or molecules etc
Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. Convert 3.01×10 23 molecules of c 2 h 6 to moles. Use this simple science molecules to moles calculator to calculate mole. So, there are approximately 0.50 moles of. 1 mole contains 6.022× 1023 number of molecules.
[Solved] How many moles are in 2.58 x 1024molecules of carbon dioxide
One mole of any substance contains 6.02×10 23 atoms/molecules. Convert 3.01×10 23 molecules of c 2 h 6 to moles. So, there are approximately 0.50 moles of. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. 1 mole contains 6.022× 1023 number of molecules.
SOLVED Calculate the number of moles of C2H6 (nC2H6) in 6.75 x 10^23
(3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Online molecules to moles calculation. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. One mole of any substance contains 6.02×10 23 atoms/molecules. So, there are approximately 0.50 moles of.
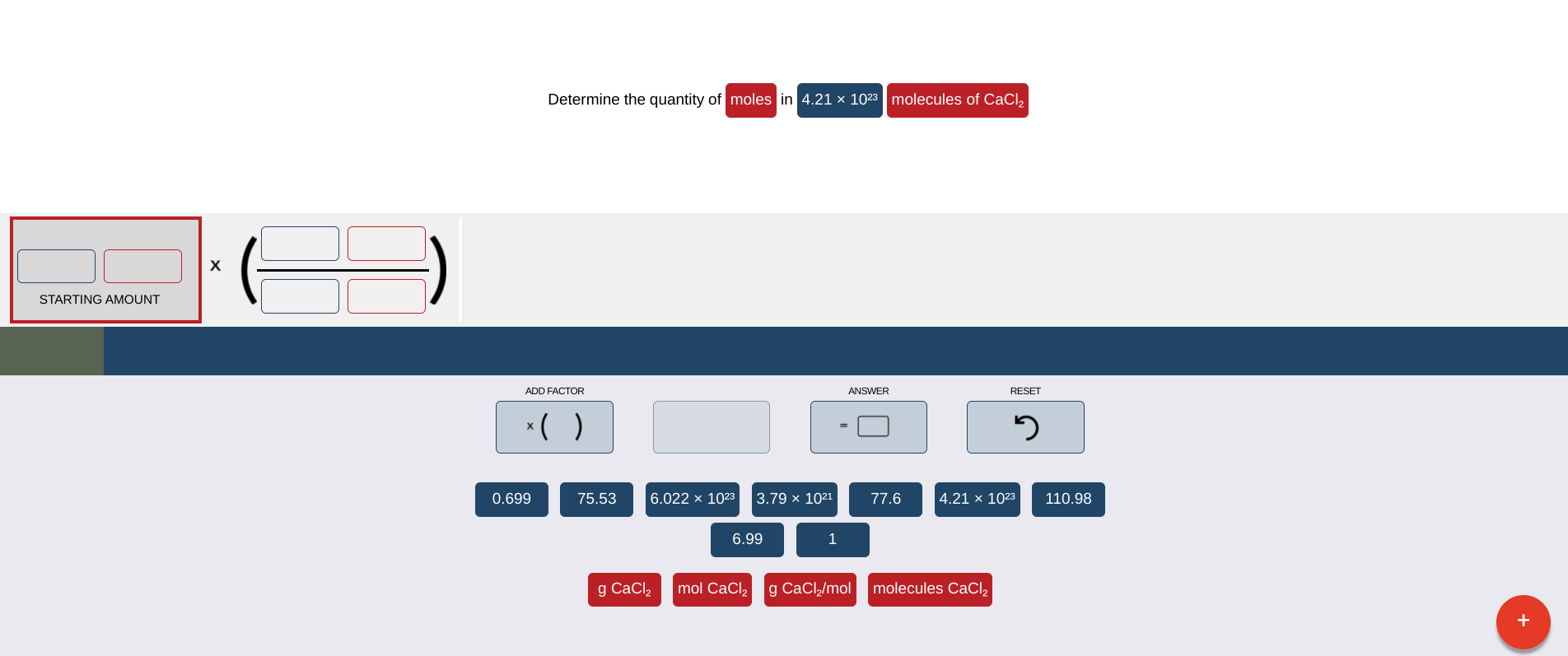
Solved Determine the quantity of moles in 4.21 x 1023
So, there are approximately 0.50 moles of. One mole of any substance contains 6.02×10 23 atoms/molecules. Online molecules to moles calculation. 1 mole contains 6.022× 1023 number of molecules. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6.
Molecules To Moles
Convert 3.01×10 23 molecules of c 2 h 6 to moles. One mole of any substance contains 6.02×10 23 atoms/molecules. Online molecules to moles calculation. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles.
Calculate The Moles Of C 2H 6 In 3.75× 1023 Molecules Of C 2H 6.
1 mole contains 6.022× 1023 number of molecules. Online molecules to moles calculation. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. One mole of any substance contains 6.02×10 23 atoms/molecules.
Convert 3.01×10 23 Molecules Of C 2 H 6 To Moles.
Use this simple science molecules to moles calculator to calculate mole. So, there are approximately 0.50 moles of. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23.