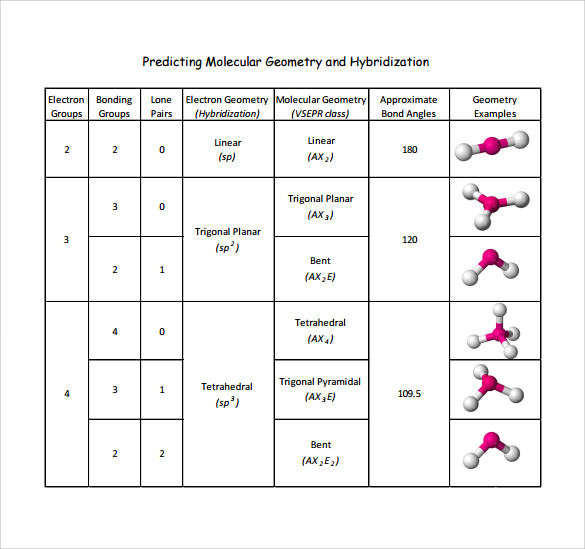
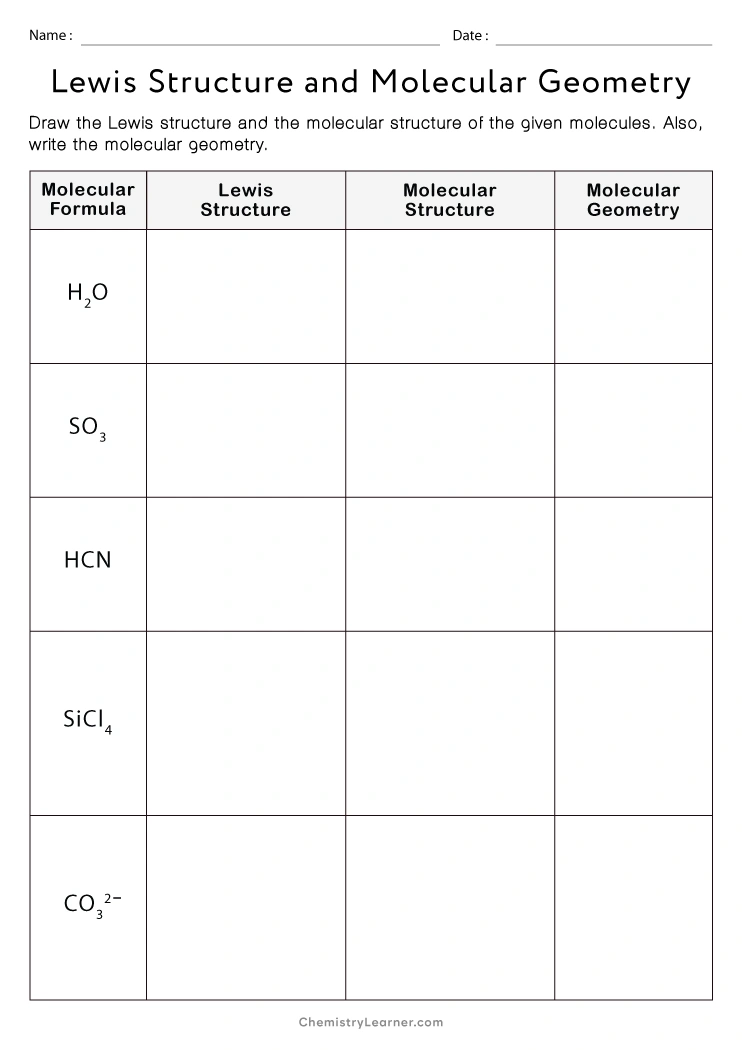
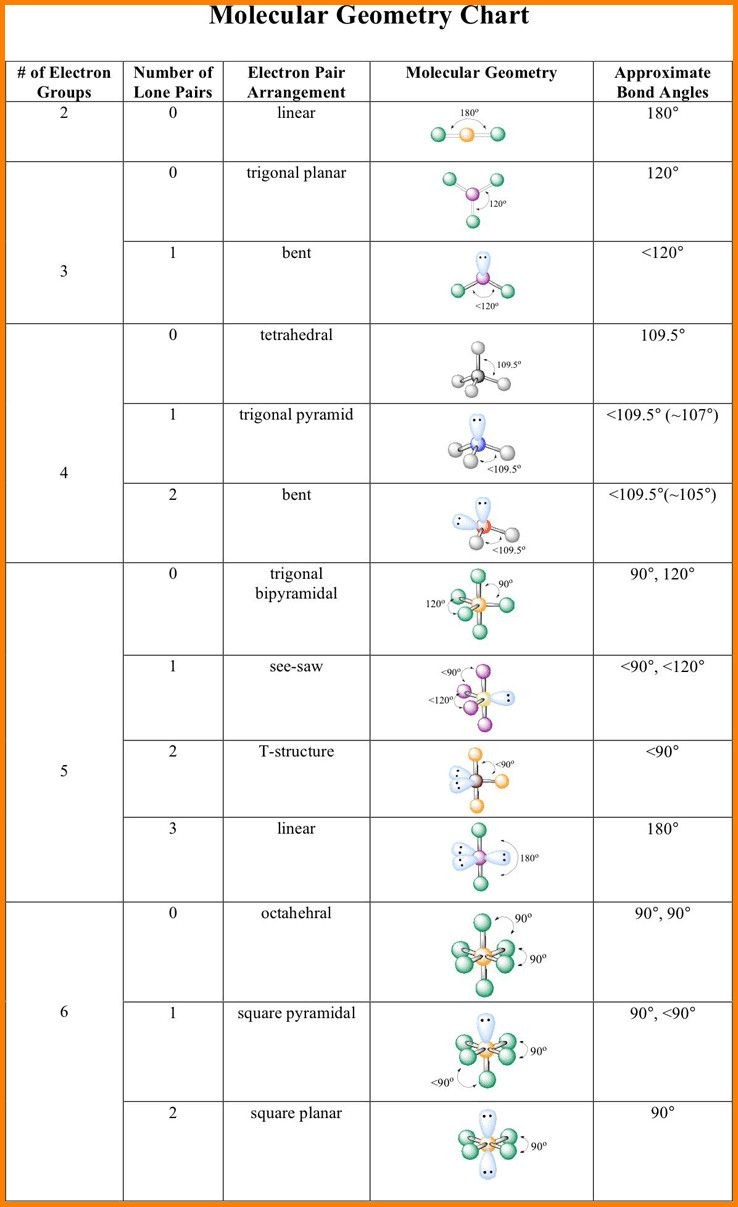
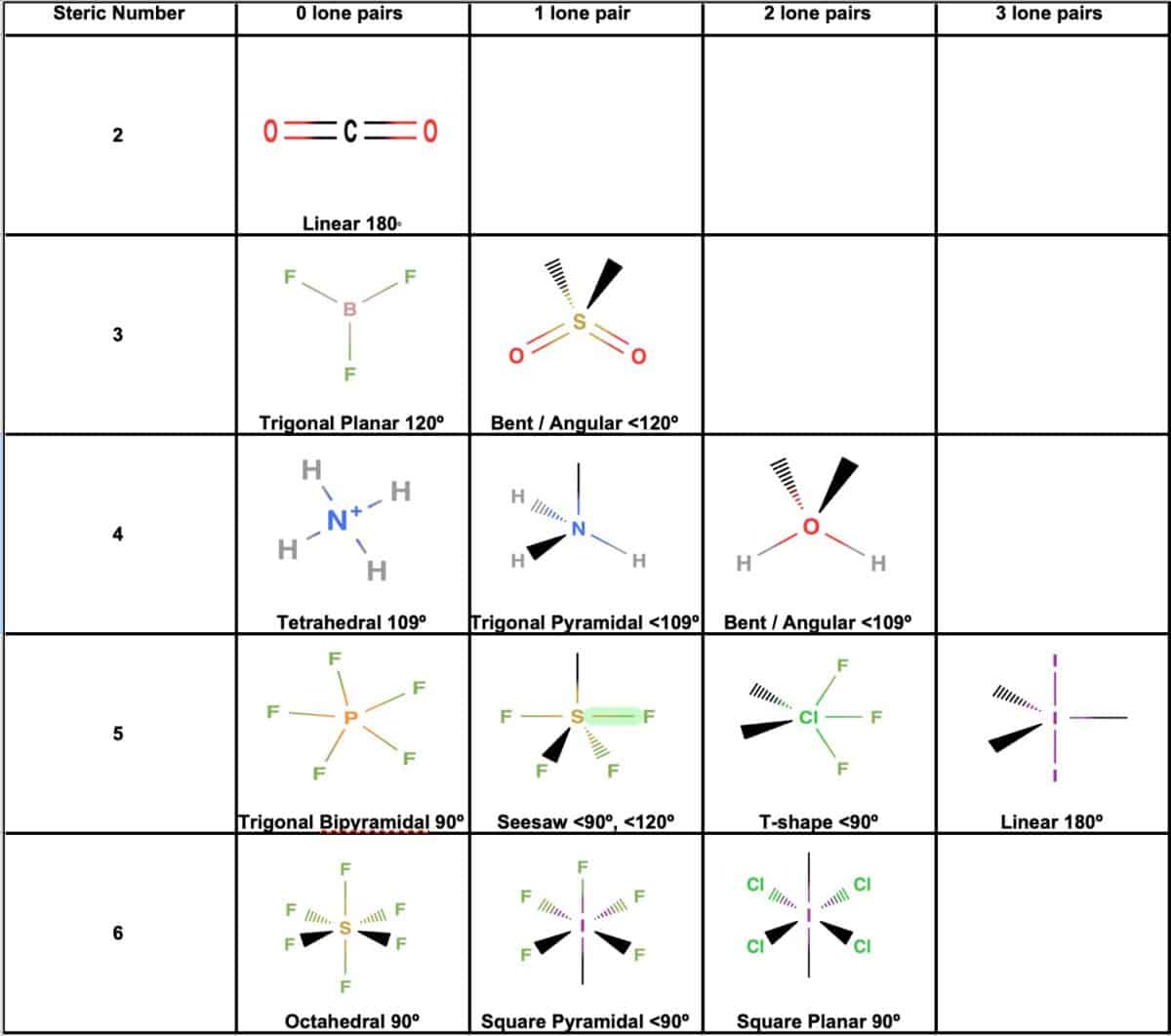
Molecular Geometry Practice Sheet - For all atoms except for hydrogen, which o. Explain why pairs of electrons around a central atom repel each other. Answer the following questions and check your answers below. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. Name the five different electronic geometries, and the eleven different. The least electronegative atom represents the central atom. Onds, then double or triple. These problems are for practice only will not be graded. Be sure you know how. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory.
Onds, then double or triple. These problems are for practice only will not be graded. Answer the following questions and check your answers below. Name the five different electronic geometries, and the eleven different. The least electronegative atom represents the central atom. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory. For all atoms except for hydrogen, which o. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. Explain why pairs of electrons around a central atom repel each other. Be sure you know how.
Be sure you know how. Answer the following questions and check your answers below. Name the five different electronic geometries, and the eleven different. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. The least electronegative atom represents the central atom. Onds, then double or triple. For all atoms except for hydrogen, which o. These problems are for practice only will not be graded. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory. Explain why pairs of electrons around a central atom repel each other.
FREE 8+ Sample Molecular Geometry Chart Templates in PDF MS Word
Explain why pairs of electrons around a central atom repel each other. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. These problems are for practice only will not be graded. Name the five different electronic geometries, and the eleven different. This quiz.
Worksheets 15 Molecular Shapes
For all atoms except for hydrogen, which o. Be sure you know how. These problems are for practice only will not be graded. Answer the following questions and check your answers below. The least electronegative atom represents the central atom.
Free Printable Lewis Dot Structure Worksheets
Be sure you know how. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. Explain why pairs of electrons around a central atom repel each other. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory..
Identify Molecular Structure Shapes
Explain why pairs of electrons around a central atom repel each other. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory. Onds, then double or triple. The least electronegative atom represents the central atom. These problems are for practice only will not be graded.
Lewis Structure Vsepr Worksheet Printable And Enjoyable Learning
The least electronegative atom represents the central atom. For all atoms except for hydrogen, which o. These problems are for practice only will not be graded. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory. Answer the following questions and check your answers below.
How To Calculate Shape Of Molecule
The least electronegative atom represents the central atom. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory. These problems are for practice only will not be graded. Onds, then double or triple. For all atoms except for hydrogen, which o.
Ap Chemistry Molecular Geometry Worksheet Kidsworksheetfun
This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory. Answer the following questions and check your answers below. Onds, then double or triple. Explain why pairs of electrons around a central atom repel each other. These problems are for practice only will not be graded.
Ap Chemistry Molecular Geometry Worksheets
Onds, then double or triple. Name the five different electronic geometries, and the eleven different. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. The least electronegative atom represents the central atom. Answer the following questions and check your answers below.
SOLUTION VSEPR Theory Molecular Shapes Worksheet Studypool
For all atoms except for hydrogen, which o. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. Explain why pairs of electrons around a central atom repel each other. These problems are for practice only will not be graded. Answer the following questions.
Xef2 Molecular Geometrymolecular Geometry Chart
Explain why pairs of electrons around a central atom repel each other. These problems are for practice only will not be graded. The least electronegative atom represents the central atom. Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. This quiz helps you.
Onds, Then Double Or Triple.
Vsepr is a model used to determine the geometry of molecules based on the number of lone electron pairs and bonds that surround a central atom. Answer the following questions and check your answers below. This quiz helps you practice identifying the molecular and electron geometry of chemical compounds using vsepr theory. Name the five different electronic geometries, and the eleven different.
These Problems Are For Practice Only Will Not Be Graded.
The least electronegative atom represents the central atom. Explain why pairs of electrons around a central atom repel each other. For all atoms except for hydrogen, which o. Be sure you know how.