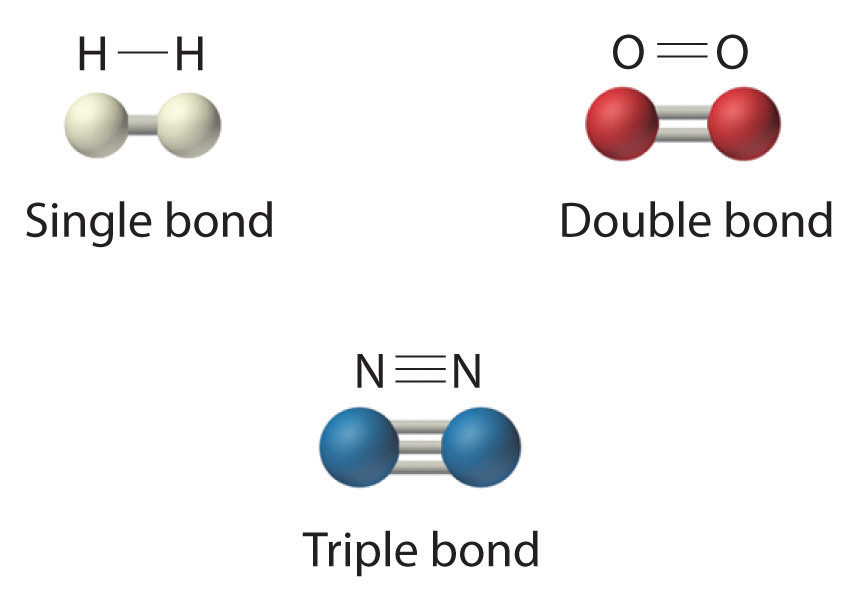
Which Two Atoms Would Typically Form A Covalent Bond - Group 5a form 3 bonds; Two different atoms can also share electrons and form covalent bonds. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Covalent bonds are formed between two atoms when both have similar tendencies to attract. Typically, the atoms of group 4a form 4 covalent bonds;
Two different atoms can also share electrons and form covalent bonds. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Covalent bonds are formed between two atoms when both have similar tendencies to attract. Group 5a form 3 bonds; Typically, the atoms of group 4a form 4 covalent bonds;
Typically, the atoms of group 4a form 4 covalent bonds; Two different atoms can also share electrons and form covalent bonds. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Group 5a form 3 bonds; Covalent bonds are formed between two atoms when both have similar tendencies to attract.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Covalent bonds are formed between two atoms when both have similar tendencies to attract. Two different atoms can also share electrons and form covalent bonds. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Typically, the atoms of group 4a form 4 covalent bonds; Group 5a form 3 bonds;
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Group 5a form 3 bonds; Covalent bonds are formed between two atoms when both have similar tendencies to attract. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Two different atoms can also share electrons and form covalent bonds. Typically, the atoms of group 4a form 4 covalent bonds;
Which covalent bond is the strongest? Socratic
Covalent bonds are formed between two atoms when both have similar tendencies to attract. Two different atoms can also share electrons and form covalent bonds. Typically, the atoms of group 4a form 4 covalent bonds; Group 5a form 3 bonds; In summary, the correct answer is that two oxygen atoms (b) form a covalent.
Covalent Bond N2
Typically, the atoms of group 4a form 4 covalent bonds; Group 5a form 3 bonds; Two different atoms can also share electrons and form covalent bonds. Covalent bonds are formed between two atoms when both have similar tendencies to attract. In summary, the correct answer is that two oxygen atoms (b) form a covalent.
Covalent Bond Definition, Types, and Examples
In summary, the correct answer is that two oxygen atoms (b) form a covalent. Two different atoms can also share electrons and form covalent bonds. Group 5a form 3 bonds; Typically, the atoms of group 4a form 4 covalent bonds; Covalent bonds are formed between two atoms when both have similar tendencies to attract.
covalent bond Definition, Properties, Examples, & Facts Britannica
In summary, the correct answer is that two oxygen atoms (b) form a covalent. Typically, the atoms of group 4a form 4 covalent bonds; Two different atoms can also share electrons and form covalent bonds. Covalent bonds are formed between two atoms when both have similar tendencies to attract. Group 5a form 3 bonds;
Covalent bond Definition, Properties, Examples, & Facts Britannica
Two different atoms can also share electrons and form covalent bonds. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Covalent bonds are formed between two atoms when both have similar tendencies to attract. Group 5a form 3 bonds; Typically, the atoms of group 4a form 4 covalent bonds;
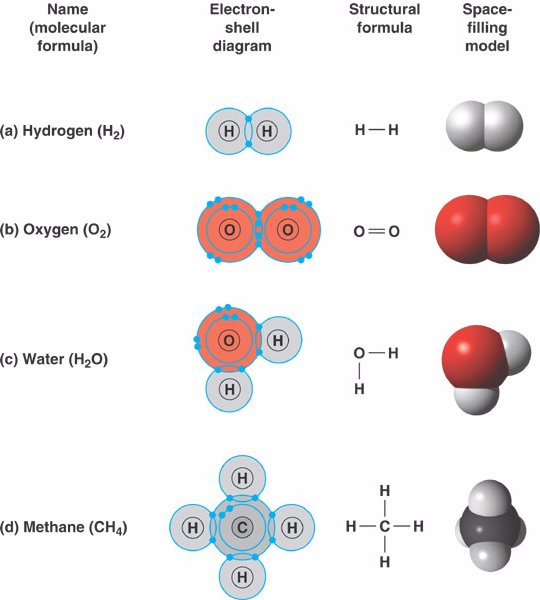
covalent_bond.html 02_11FourCovalentBonds_A.jpg
Typically, the atoms of group 4a form 4 covalent bonds; Covalent bonds are formed between two atoms when both have similar tendencies to attract. Two different atoms can also share electrons and form covalent bonds. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Group 5a form 3 bonds;
How hydrogen atoms share valence electrons to form covalent bond and
Covalent bonds are formed between two atoms when both have similar tendencies to attract. Group 5a form 3 bonds; Two different atoms can also share electrons and form covalent bonds. Typically, the atoms of group 4a form 4 covalent bonds; In summary, the correct answer is that two oxygen atoms (b) form a covalent.
Covalent Bonding (ALevel) ChemistryStudent
Typically, the atoms of group 4a form 4 covalent bonds; Covalent bonds are formed between two atoms when both have similar tendencies to attract. In summary, the correct answer is that two oxygen atoms (b) form a covalent. Two different atoms can also share electrons and form covalent bonds. Group 5a form 3 bonds;
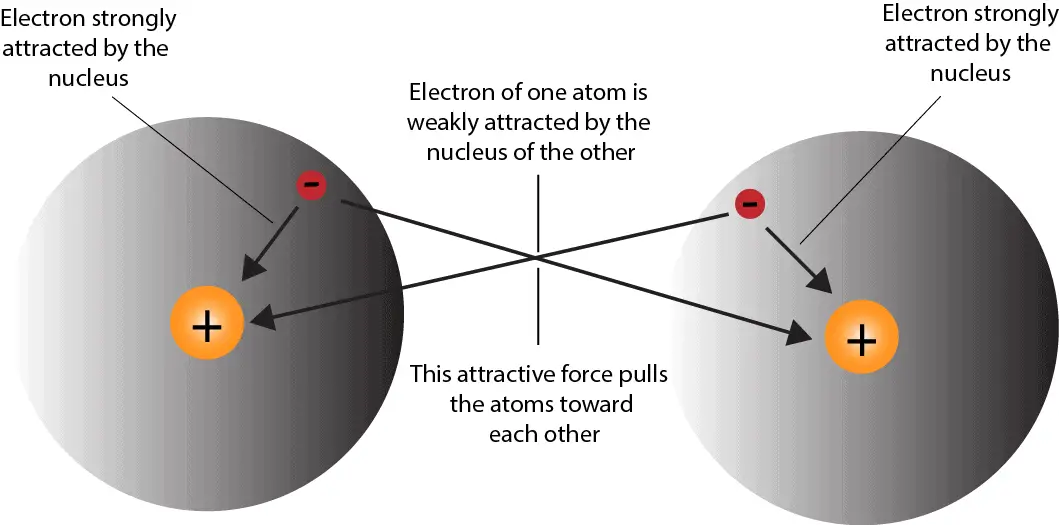
Two Different Atoms Can Also Share Electrons And Form Covalent Bonds.
Group 5a form 3 bonds; Covalent bonds are formed between two atoms when both have similar tendencies to attract. Typically, the atoms of group 4a form 4 covalent bonds; In summary, the correct answer is that two oxygen atoms (b) form a covalent.